Today's KNOWLEDGE Share:
Yellowing of Polycarbonate
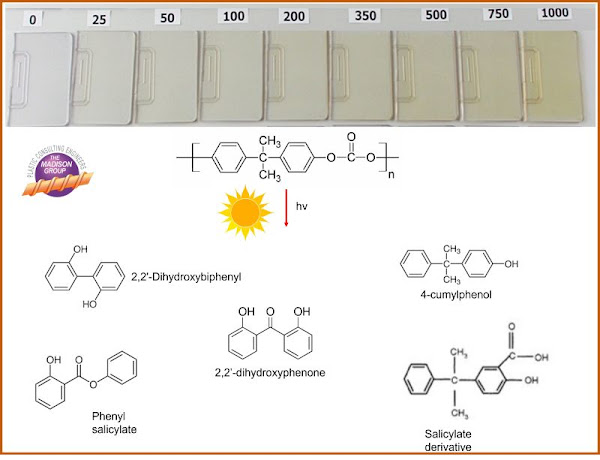
Because of the molecular structure of polycarbonate, the polymer is susceptible to yellowing through a variety of degradation mechanisms. Discoloration of polycarbonate can occur through oxidation, either during processing or as a result of elevated temperature exposure in air. Yellowing can also take place through sterilization, such as autoclaving, radiation, or ethylene oxide. Further, polycarbonate is highly susceptible to yellowing through ultraviolet radiation (UV) exposure.
When polycarbonate is exposed to ultraviolet radiation as sunlight, yellowing can take place rapidly. This discoloration is principally a surface phenomenon, approximately 25 micrometers deep, due to the penetration depth of the UV radiation into the polycarbonate.
Yellowing within polycarbonate occurs as a result of the formation of degradation breakdown products that absorb in the yellow range of the visible light spectrum. This includes substituted ortho-quinones, quinone methides, phenones and phenone derivatives, and bisphenol-A derivatives. Analytical techniques, including gas chromatography-mass spectroscopy (GC-MS) and Fourier transform infrared spectroscopy (FTIR), have been used to study the degradation mechanisms and characterize the breakdown products.
The yellowing of the polycarbonate is accentuated by the high level of transparency, which results in a strong yellow hue.
The effects of UV exposure, as well as oxidation and hydrolytic degradation, can be assessed through accelerated aging followed by color evaluation and physical/mechanical testing. Contact me to see how this can benefit you.
Souce:The Madison Group
Visit MY BLOG http://polymerguru.blogspot.com
#plastics #failureanalysis #uv #degradation #discoloration #yellowing #polycarbonate #testing #ftir #phenone




No comments:
Post a Comment