Today's KNOWLEDGE Share:
Fourier transform infrared spectroscopy (FTIR) is a fundamental tool for the qualitative compositional analysis of polymeric materials. It allows the user to identify the material being tested. In order to properly interpret the results of FTIR, the key considerations regarding spectral band formation must be understood.
FTIR bombards with infrared energy, and certain frequencies are absorbed by the sample and others transmitted. The frequencies at which the material absorbs infrared energy correspond to molecular vibrations that are produced within the sample. The infrared spectrum representing a material consists of absorption bands associated with discrete functional groups, the building blocks that make up the molecule. The frequency at which a functional group absorbs is based on a number of factors:
Atomic weight of the bonded species: Frequency decreases (lower cm-1) with increasing atomic weight.
Bond energy: Frequency increases (higher cm-1) with increasing bond energy.
Surrounding molecular structure: The adjacent molecular structure changes the vibrations of the subject bond, for example conjugation lowers the frequency (lower cm-1).
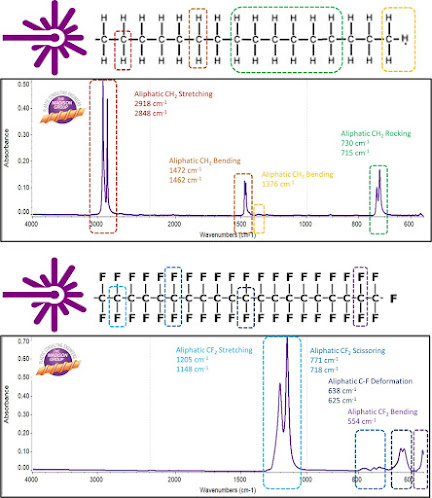
An important implication of this is the absorption bands associated with halogenated polymers, particularly fluoropolymers. The C-X stretching and bending frequencies occur at lower frequencies (lower cm-1) in C-I < C-Br < C-Cl < C-F < C-H. This is primarily due to the effects of the molecular mass bond energies. Because of this shift, the presence of a halide is often difficult to confirm by means of the infrared spectroscopy.
I have illustrated the spectral band shift between analogous C-H bond and C-F bands in polyethylene and polytetrafluroethylene, respectively, in the color-coded graphic below.
Source:Jeffrey A. Jansen




No comments:
Post a Comment