Today's Knowledge Share:
Healing Halogens
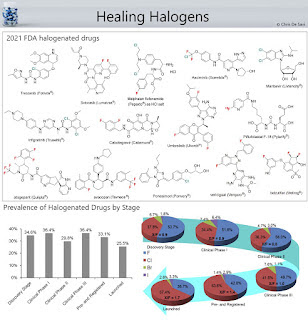
A significant number of drugs are halogenated.
Typically, insertion of halogen atoms on hit or lead compounds is performed to occupy the binding site of molecular targets in order to improve the drug−target binding affinity and/or reduce metabolism. 14 out of the 50 molecules approved by the FDA in 2021 contain halogens.
The introduction of F onto a chemical scaffold is able to infer changes that affect the physicochemical properties and the conformation of a molecule. Being the most electronegative element in the periodic table, F plays an important role in the modulation of pKa of neighboring functionalities. Substituting F for H on aromatic groups is also well known to improve metabolic stability.
Lipophilicity is also affected by the addition of F onto aliphatic and aromatic scaffolds. The monofluorination or trifluoromethylation of saturated alkyl groups usually decreases the lipophilicity due to the strong electron-withdrawing capabilities of fluorine. F-arenes are more lipophilic than des-F ones due to the low polarizability of the C-F bond. The installation of a fluorine atom in the ortho-position to an NH function on the aromatic ring is often used to enhance membrane permeability.
From a conformational perspective, the addition of one single F has a reduced steric effect, leaving mostly unchanged the interaction with the receptor site if compared to the same interaction with a molecule bearing an H atom in the same position. This can be explained by the similar van der Waals radii of F and H: 1.47 Å and 1.20 Å, respectively.
The commonly used trifluoromethyl group is sterically more demanding and almost equivalent to an ethyl group. The highly electronegative nature of fluorine makes it a hydrogen bond acceptor from HBD but does not establish as good of halogen bonds as Cl and Br, because it does not typically feature a σ-hole. Another important use is that fluorinated functionality can be incorporated into endogenous substrates or ligands through 19F-markers to investigate protein functions.
Cl is greater in size than F, and the C–Cl bond is stable enough that it allows its insertion in diverse heterocyclics. Cl is a better halogen bond acceptor compared to F. The most important impact of a nonreactive Cl atom on the biological activity of many compounds comes when it is a substituent on an aromatic, heteroaromatic or olefinic moiety. In these cases, the steric and/or electronic effects of the chlorine substituents lead to local electronic attraction or repulsion and/or to steric interference with any surrounding amino acids of target proteins. The Cl atom is often viewed as isosteric and has similar physicochemical properties to the methyl grou.
>250 FDA-approved chlorine-containing drugs are available on the market (as of 2019).




No comments:
Post a Comment