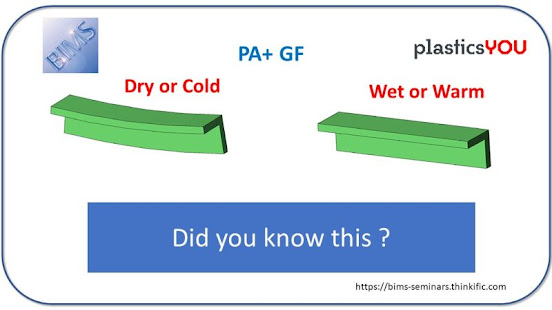
A team of chemists at Purdue University led by Jonathan Wilker, professor of chemistry in the College of Science and of materials engineering, aims to develop a completely #sustainable #adhesive system.
“Our current adhesives create all sorts of environmental problems,” Wilker said. “Almost all glues are petroleum-based and do not degrade. The bonded materials in our products stay stuck together. Consequently, we cannot recycle many of the materials that we put into our recycling bins. Discarded products will sit in landfills for centuries and, sometimes, contribute to ocean microplastics.
Nature-Inspired Innovation
Wilker and his lab have spent years studying the science of sticky substances, analyzing marine animals that adhere, like mussels and oysters, and trying to create better, sustainable, affordable adhesives that work as well as any glue from the hardware store. He has a drawer of those commercial glues in his lab, which give off a strong and familiar smell.
“Those volatile petrochemicals in these glues can be toxic, which is a further problem with current technologies,Wilker said. One example is the common building material plywood, which is formed of wood pieces held together with formaldehyde-based adhesives. Newly built houses are off-gassing formaldehyde, exposing residents to this carcinogen.
Making Adhesives Safer and Stronger:
These substances are harmful both to the environment and to human health. However, people and companies are accustomed to using traditional adhesives; they’re strong, easy to produce and relatively inexpensive. Any new adhesive must work at least as well as traditional products, which is why Wilker keeps that drawer around: to test them, side by side against innovative substances.
“By studying how nature makes adhesives, we are learning how to design new technologies for our future society,” Wilker said. “Given all of the problems generated by current glues, we feel an obligation to create something better. Ideally, new adhesives will be bio-based and nontoxic. Strengths should be as high as current products. Then we would like to bond them strongly when needed and also be able to take the substrates apart when wanted. Further design constraints that we grapple with, in order to have impact, are costs needing to be low and having all starting compounds available at large scales.
After a series of experiments on a range of different biologically sourced and sustainable ingredients, the team settled on epoxidized soy oil for a main component. Epoxidized soy oil is already produced globally on a massive scale. For their work, the smallest container that they could purchase was a 55-gallon drum of the substance. Since each experiment uses just a little epoxidized soy oil, the level in their drum has dropped only a few inches after several years of testing.
Eco-Adhesives for a Sustainable Tomorrow
Wilker and his team added the epoxidized soy oil to malic acid, a compound most known for giving apples their tart flavor. Then they added tannic acid, to provide an aspect of the chemistry that mussels use for attaching themselves to rocks and each other. Tannic acid is a component of tannins, common in trees, red wine and black tea. Those three ingredients added up to an adhesive that is inexpensive, effective, scalable, practical to produce and completely sustainable.
“If you combine these components under the right conditions, adhesives can be made that are as strong as epoxies,” Wilker said. Epoxies are generally considered to be the highest performance class of adhesives. “All of the components are bio-based, safe and already available at train car scales. A bonus is that the adhesive is easy to make. Basically, you can mix and heat the components.” Other bio-based compounds can also be used with epoxidized soy oil, generating an entire family of new sustainable adhesives.
To test the adhesive’s performance, the scientists bonded together objects — wood, plastics or metals — and then used an instrument for breaking the bonds and measuring forces. In many cases, their new adhesives held up well, sometimes performing similarly to, or even better than, traditional toxic adhesives such as a superglue and an epoxy. Further research will refine the system and work to maximize societal and environmental impacts in areas ranging from medical innovations to industrial materials to packaging. Their team’s innovations may pave the way to a more sustainable system for holding the world together.
Wilker disclosed his adhesives to the Purdue Innovates Office of Technology Commercialization, which has applied for a patent to protect the intellectual property. This research was supported by the Office of Naval Research.
Source: Purdue University/specialchem
Follow: http://polymerguru.blogspot.com