The Yokohama Rubber Co., Ltd., has announced that the results of joint
research projects conducted since 2013 with two universities in
Thailand, a major producer of natural rubber, were recently presented at
The International Polymer Conference of Thailand 2018 (PCT-8).
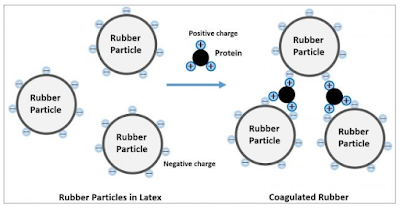
The joint research projects were conducted with researchers at Mahidol University and Prince of Songkla University. The research with Mahidol University succeeded in analyzing proteins contained in sap (latex), the base raw material for natural rubber, and identifying the proteins deeply involved in natural rubber biosynthesis. The research deepens the understanding of the biosynthesis of natural rubber, making it possible to accelerate research related to quality and production.
The research conducted at Mahidol University entailed the extraction and nano-level analysis of proteins from fresh latex and seedlings from Para rubber trees. The analysis covered more than 800 kinds of proteins contained in latex, some of which were found to be related to natural rubber biosynthesis and stress resistance. In addition, by comparing proteins from different varieties of Para rubber trees, the researchers were able to identify the proteins that promote biosynthesis and the proteins that inhibit biosynthesis. The proteins are expected to be used as biomarkers of biosynthesis.
The research at Prince of Songkla University was fundamental research on natural rubber that focused on analyzing the differences in latex related to different seasons and regions, different varieties and different processing methods. The research also evaluated the presence or absence of changes in the physical and chemical properties of rubber over long periods of time. To date, natural rubber has been a very stable material, from its composition to its physical properties, and it has been highly resistant to external factors.
Natural rubber is a raw material made from latex taken from Para rubber
trees. It is one of the main raw materials used in automotive tires,
accounting for about 30% of tires made. However, natural rubber’s
production is concentrated in Southeast Asia, which exposes large-scale
production to risks from abnormal weather and disease. Expecting tire
demand to expand in the future, Yokohama Rubber regards the improvement
of the quality of natural rubber and the promotion of technological
development contributing to stable production as an important corporate
duty. Accordingly, the Company plans to use the results of this research
to promote the maintenance and development of natural rubber
plantations.
Source: Yokohama Rubber Co
The joint research projects were conducted with researchers at Mahidol University and Prince of Songkla University. The research with Mahidol University succeeded in analyzing proteins contained in sap (latex), the base raw material for natural rubber, and identifying the proteins deeply involved in natural rubber biosynthesis. The research deepens the understanding of the biosynthesis of natural rubber, making it possible to accelerate research related to quality and production.
Evaluating Physical and Chemical Properties of Rubber
The research conducted at Mahidol University entailed the extraction and nano-level analysis of proteins from fresh latex and seedlings from Para rubber trees. The analysis covered more than 800 kinds of proteins contained in latex, some of which were found to be related to natural rubber biosynthesis and stress resistance. In addition, by comparing proteins from different varieties of Para rubber trees, the researchers were able to identify the proteins that promote biosynthesis and the proteins that inhibit biosynthesis. The proteins are expected to be used as biomarkers of biosynthesis.
The research at Prince of Songkla University was fundamental research on natural rubber that focused on analyzing the differences in latex related to different seasons and regions, different varieties and different processing methods. The research also evaluated the presence or absence of changes in the physical and chemical properties of rubber over long periods of time. To date, natural rubber has been a very stable material, from its composition to its physical properties, and it has been highly resistant to external factors.
Enhancing Maintenance and Development of Natural Rubber Plantations:
Natural rubber is a raw material made from latex taken from Para rubber
trees. It is one of the main raw materials used in automotive tires,
accounting for about 30% of tires made. However, natural rubber’s
production is concentrated in Southeast Asia, which exposes large-scale
production to risks from abnormal weather and disease. Expecting tire
demand to expand in the future, Yokohama Rubber regards the improvement
of the quality of natural rubber and the promotion of technological
development contributing to stable production as an important corporate
duty. Accordingly, the Company plans to use the results of this research
to promote the maintenance and development of natural rubber
plantations.
The Yokohama Rubber Group has positioned “Promotion of CSR activities throughout the value chain”
as one of the important issues of the Group’s corporate social
responsibility (CSR) activities. Accordingly, in addition to the above
joint research projects on natural rubber, the Group is engaged in
activities that will contribute to sustaining farmlands. These
activities have included biodiversity surveys on natural rubber
plantations and promoting widespread use of an “agroforestry farming method”
that contributes to more stable income for rubber tree growers by
planting bamboo, fruit trees and other plants in natural rubber forests.
Source: Yokohama Rubber Co