As many questions related to materials engineering, to answer that we have to understand the unit cell structure of the material, in this case, graphite.
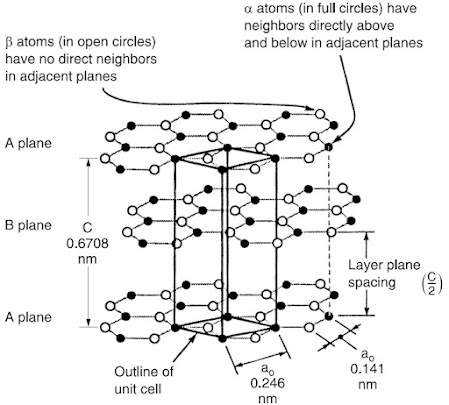
The crystal structure of graphite, consists of sp2 hybridized carbon atoms arranged two-dimensionally in a honeycomb structure in the x-y plane. The layers, termed graphene layers, are stacked parallel to each other in a 3D structure. The most common stacking sequence of the layer planes is the hexagonal form with an ABABAB packing sequence. This way, some atoms (α) have neighbors directly above and below in adjacent planes, while others (β) don’t. The bonding between the layers is van der Waals bonding, so the carbon layers can easily slide with respect to one another.
Due to the difference between the in-plane and out-of-plane bonding, graphite has a high modulus of elasticity parallel to the plane and a low modulus perpendicular to the plane. Thus, graphite is highly anisotropic. The high modulus of a carbon fiber stems from the fact that the carbon layers, though not necessarily flat, tend to be parallel to the fiber axis.
Source:#managingcomposites




No comments:
Post a Comment