Today's KNOWLEDGE Share: Corner Deformation
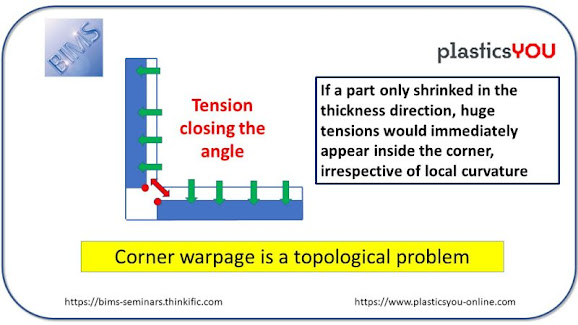
Today's KNOWLEDGE Share Corner Deformation I thought I would post a more complete answer to this issue related to corner deformation. In the paper we published in 2003,we used both experiments and simulations to show that the vastly dominant effect in angular deformation of molded curved/angular shapes does NOT originate from a cooling issue and is little sensitive to fillet radius. Hopefully, my drawing above should convince you that the only way to have curvature preserved after shrinkage is to have exactly the same shrinkage in the z thickness direction and in the x-y part plane direction. In molding this is never the case : - For filled polymers, a much higher CLTE in z derives from the nearly total lack of fibers in the z direction - For unfilled polymers, the combination of x-y high mold constraints, freedom to shrink in z, and relaxation of x-y in-mold stresses, always leads to higher z shrinkage vs.in-plane shrinkage. Source:Vito Leo Visit MY BLOG http: