Today's KNOWLEDGE Share:
Failure Analysis of Electrical Connectors
The metal leads on low voltage electrical connectors cracked during outdoor exposure testing. Initially, a metallurgical failure analysis was performed, concluding that the leads failed due to pitting and stress corrosion cracking (SCC).
As part of the metallurgical analysis, the lead wires were identified as a 65% Cu/35% Zn yellow brass with an exterior silver plating and a nickel underplating. These results were verified as part of the continued evaluation I performed through SEM-EDS analysis and elemental mapping.
The plastic base material was specified as an unfilled polypropylene, formulated with tetrabromobisphenol A bis(dibromopropyl ether) a brominated flame retardant; and antimony oxide a synergistic flame retardant additive. This was confirmed through Fourier transform infrared spectroscopy (FTIR) and EDS.
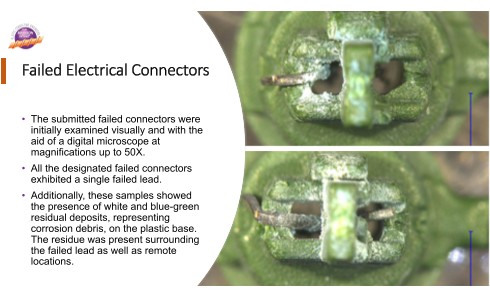
Visual and microscopical examinations confirmed the cracked leads. The connectors also showed the presence of white and blue-green deposits, as corrosion debris, surrounding the failed leads.
The connectors were inspected via scanning electron microscopy (SEM) and tested using energy dispersive X-ray spectroscopy (EDS). The plastic core showed a high concentration of carbon; moderate levels of bromine, oxygen, and antimony; and a trace of chlorine. The carbon was principally present as polypropylene. Some of the carbon, the bromine, the oxygen, the antimony, and the chlorine represented the flame retardant package.
The SEM examination confirmed the presence of corrosion debris, as a mud cracked morphology, consistent with the deposition of metallic corrosion product. Elemental analysis of these surface deposits showed high concentrations of copper and zinc, and a substantial increase in the level of oxygen. The presence of bromine was also indicated within the corrosion deposits.
The SEM examination of the plastic bases adjacent to the failed leads revealed needle-like particles, with relatively high concentrations of oxygen and bromine. The antimony content within the needles was not elevated relative to the base plastic. These results and the form of the particles indicated that the needles represented brominated flame retardant that had migrated from the base plastic onto the surface - a phenomenon referred to as bloom.
The needle-like particles observed on the failed connectors were identified as the brominated flame retardant, which had bloomed to the surface during the exposure testing. The brominated flame retardant acted as a corrosive agent in conjunction with the connector lead wires. Under conditions of weathering and exposure to moisture, it is possible that the brominated flame retardant produced degradation products that would be even more corrosive to the lead wires.
Source:The Madison Group
Visit MY BLOG http://polymerguru.blogspot.com
#plastics #failureanalysis #ftir #polymers #polypropylene #sem #fractography #electrical #corrosion