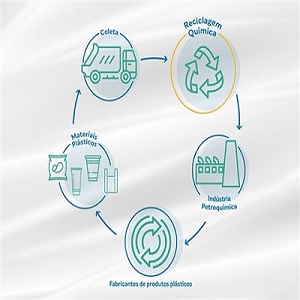
Seeking solutions that contribute to the circular economy and to sustainable development, Braskem
announces new partnerships for the development of chemical recycling.
It will be focused on transforming post-consumer plastics, such as
grocery bags and packaging films for snacks and cookies, once again into
chemical products that can be used by various different value chains
and will benefit the general public. Braskem has applied its knowledge
and commitment once again to move the industry towards the circular
economy.
The partnerships seek to further research into technologies that can transform plastics that are more difficult to be recycled mechanically into new chemical products. The research is being conducted in partnership with:
"As we strive to reach a true circular economy, we recognize the challenges and limitations posed by traditional recycling technologies. Braskem is committed to developing, implementing and offering sustainable solutions. Chemical recycling and its potential to overcome all these challenges and limitations will enable us to achieve this goal. We are accelerating these efforts through partnerships and collaboration with other companies that think like we do in order to reach these targets as soon as possible," explained Gus Hutras, head of Process Technology at Braskem.
This new technological route complements the initiatives that Braskem has recently undertaken to contribute to the Circular Economy, which is a production concept that involves reducing, reusing, recuperating and recycling materials to create a sustainable cycle from the production phase to the reintegration of materials in a new production process.
"These studies in chemical recycling uphold the principles of Braskem, which drives innovation to develop sustainable solutions. Every day, we want to create businesses and initiatives that increase the value of plastic waste," said Fabiana Quiroga, head of Recycling. "The advantage of chemical recycling lies in its capacity to process and transform plastic waste back into a raw material that can be used to make new plastics," she added.
To add value to materials made from recycled resins, Braskem has maintained, since 2015, the Wecycle platform, which combines the need for proper disposal with the market's demand for sustainable raw materials. The platform works to develop businesses and initiatives that add value to plastic waste through partnerships, which enhances the development of products, solutions and processes that involve all links of the plastics recycling chain by supporting businesses and actions involving recycling.
Braskem, the Americas' largest resin producer and the world's leading biopolymer producer, has defined a series of global initiatives to boost the Circular Economy in the production chain of manufactured plastic goods. Entitled "Braskem's Positioning for the Circular Economy," the document establishes:
Braskem invites its clients and other stakeholders to join forces. The initiatives also include encouraging consumers to get involved in recycling programs through educational actions focusing on conscientious consumerism, the use of life cycle assessment tools and support for actions that improve solid waste management to prevent the disposal of debris in marine environments.
Source: Braskem
Research into Technologies Transforming Plastics
The partnerships seek to further research into technologies that can transform plastics that are more difficult to be recycled mechanically into new chemical products. The research is being conducted in partnership with:
- Polymer Engineering Laboratory (EngePol) at the Alberto Luiz Coimbra Institute of Graduate Studies
- Research in Engineering of the Federal University of Rio de Janeiro (COPPE/UFRJ)
- SENAI Institute for Innovation in Biosynthetics (SENAI CETIQT)
- Cetrel (an environmental service company that started its activities in 1978 jointly with manufacturers located in the Camaçari Petrochemical Complex)
"As we strive to reach a true circular economy, we recognize the challenges and limitations posed by traditional recycling technologies. Braskem is committed to developing, implementing and offering sustainable solutions. Chemical recycling and its potential to overcome all these challenges and limitations will enable us to achieve this goal. We are accelerating these efforts through partnerships and collaboration with other companies that think like we do in order to reach these targets as soon as possible," explained Gus Hutras, head of Process Technology at Braskem.
Technological Route Complementing Braskem’s Initiatives
This new technological route complements the initiatives that Braskem has recently undertaken to contribute to the Circular Economy, which is a production concept that involves reducing, reusing, recuperating and recycling materials to create a sustainable cycle from the production phase to the reintegration of materials in a new production process.
"These studies in chemical recycling uphold the principles of Braskem, which drives innovation to develop sustainable solutions. Every day, we want to create businesses and initiatives that increase the value of plastic waste," said Fabiana Quiroga, head of Recycling. "The advantage of chemical recycling lies in its capacity to process and transform plastic waste back into a raw material that can be used to make new plastics," she added.
To add value to materials made from recycled resins, Braskem has maintained, since 2015, the Wecycle platform, which combines the need for proper disposal with the market's demand for sustainable raw materials. The platform works to develop businesses and initiatives that add value to plastic waste through partnerships, which enhances the development of products, solutions and processes that involve all links of the plastics recycling chain by supporting businesses and actions involving recycling.
Braskem's Initiatives to Promote the Circular Economy
Braskem, the Americas' largest resin producer and the world's leading biopolymer producer, has defined a series of global initiatives to boost the Circular Economy in the production chain of manufactured plastic goods. Entitled "Braskem's Positioning for the Circular Economy," the document establishes:
- Initiatives for forging partnerships with clients to conceive new products that will extend and facilitate the recycling and reuse of plastic packaging, especially single-use packaging
- It also establishes higher investments in new resins derived from renewable resources, such as Green Plastic made from sugar cane, and support for new technologies, business models and systems for collecting, picking, recycling and recovering materials
Braskem invites its clients and other stakeholders to join forces. The initiatives also include encouraging consumers to get involved in recycling programs through educational actions focusing on conscientious consumerism, the use of life cycle assessment tools and support for actions that improve solid waste management to prevent the disposal of debris in marine environments.
Source: Braskem